Phosphorus is a nutrient that is greatly needed for high yields in crop production. It stimulates root development, increases stalk and stem strength, improves flower formation and seed production, improves crop quality, and supports development throughout the entire life cycle of the plant.
Phosphorous has also been receiving a lot of attention lately in the ag industry with the emphasis being on using the 4Rs best management practices to reduce or eliminate it moving into the Great Lakes. Many growers in the province still do not soil sample properly. If a farm is not soil sampled correctly, understanding how much phosphorous is in your soil is simply a guess. Having large quantities of phosphorus in your soil doesn’t mean it will all be available to a crop.
A plant will take in phosphorus through three different ways. In the soil including interception, mass flow and diffusion. The majority typically is taken up through diffusion which is the movement of ions from areas of high concentration to areas of low concentration. Phosphorus is very insoluble, so movement is very slow. Placement and maintaining good soil levels of phosphorus are essential. An example of this is if you have loam soil, Phosphorus must be less than ¼’’ from the root to be taken up. Phosphorus does not readily leach out of the root zone. The loss of Phosphorus mainly stems from erosion and run-off of fertiliser or soil-adsorbed P.
Many growers do not know that phosphorus can easily become tied up and unavailable in their soil.
Two important things to remember about phosphorus is:
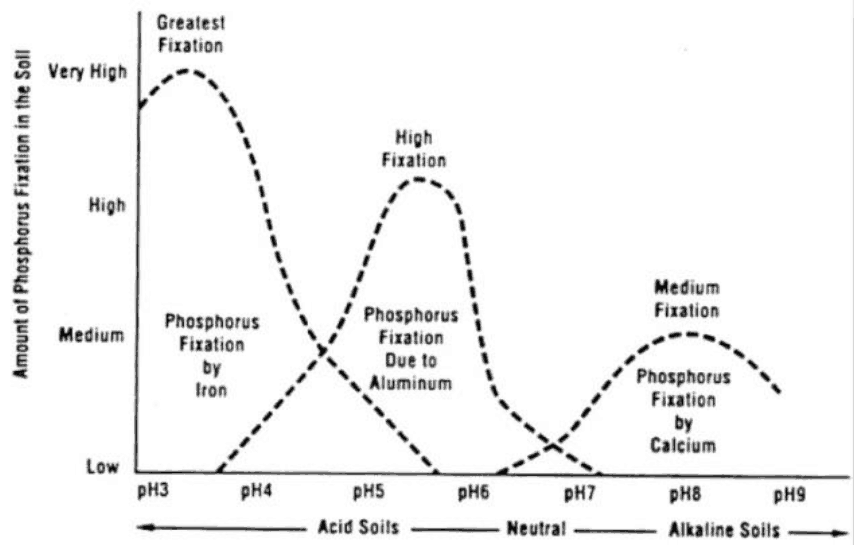
- As the chart below indicates, high pH levels with anything above 7.3 has calcium that can combine chemically with phosphorus, making a compound that is insoluble and that crops can’t access.
- Lower soil pH that is below 6.3 can have iron and aluminium and manganese begin to tie up the phosphorus also making it unavailable to the plant, as the chart below indicates.
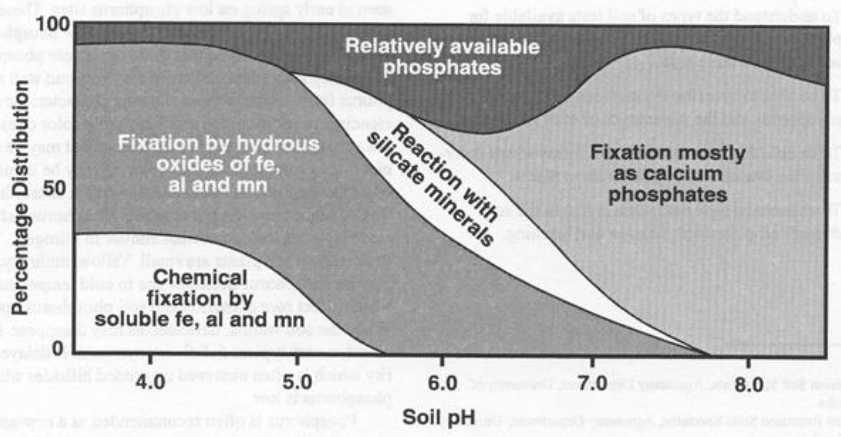
Phosphorus can exist in both organic and inorganic forms. In organic materials, the phosphorus is released by mineralization which occurs when microorganisms break down soil organic matter. For inorganic phosphorus, when phosphorus reacts with Iron, Aluminum and Calcium, it will create a product that is not very soluble and this is considered to be fixed or tied up.
There are several ways to protect and manage your phosphorus from becoming tied up in your soils:
- Band your Phosphorus.This keeps areas concentrated in the soil allowing less to become tied up then if it was just broadcasted on the top or broadcasted and incorporated.
- Take advantage of products that protect your Phosphorus from getting tied up. Using a product such as Avail from the Andersons can be a great option as it reduces fixation of phosphorus in the soil.
- Cold soil and starters.If organic matter is a source of P, then it will release slowly if the soil is cool and wet. Roots have a hard time absorbing P when the soil is cold. That is why starters with P close to the seed is so important.
- pH counts. We know that Phoshorus is most available between a pH of 6.5-6.8 but it can be hard to keep our soils exactly in that range. Accurate, proper, and timely soil sampling can make sure lime is being applied to correct to the pH, or acidifying products are used to lower the pH.
- Mycorrhizae to the rescue. These are fungi that live in the soil and invade plant roots. They can provide supplementary P to the plant. These fungi move out over the soil and absorb many minerals such as Phosphorus, and pass that on to the plant.
A phosphorus deficient corn plant (Purdue University).
Myths about phosphorus
- MYTH: Orthophosphate is better than polyphosphate because it is in the plant available form.
- Polyphosphate ions are readily converted to orthophosphate ions in the presence of soil moisture. If the soil temperatures are normal the conversion can be completed in days with normal soil temp. Trials done show that fertilizer applied in the different forms clearly show that a pound of P is a pound of P.
- MYTH: Liquid Phosphate is more mobile in the soil.
- Phosphate will not move very far at all in the soil regardless of fertilizer form (liquid or granular.)
- MYTH: Liquid Phosphate is available throughout the entire growing season.
- Phosphate fertilizer in any form can get tied up quickly. Only 10-30% of applied phosphate fertilizer is available during the season it was applied in. It is important to remember that seed placed P fertilizer will always be more efficiently available compared to any broadcast that is spread on the field.
- Mainly a MYTH: Elemental Sulfur acidifies the soil and frees up phosphate for the plant.
- While it is true that elemental sulfur can acidify the soil or acidify a part of the soil locally, it has not been proven to be effective for improving phosphate availability.
Availability factors:
Several factors come into play when you are dealing with phosphorus and making it available to the plant.
Aeration and compaction
If water levels are low, absorption by roots is low.
Moisture
Diffusion needs moisture, excess limits water.
P level of the soil
More P concentration to move around.
Amount and type of clay
More P concentration to move around.
Time and method of application
The longer time it is applied before a crop the more chance it has to bind with the soil. Method determines how far it will be placed from the roots.
Other nutrients
Levels of other nutrients can affect uptake. The longer time it is applied before a crop the more chance it has to bind with the soil. Method determines how far it will be placed from the roots.
Soil pH
Most available at 6.5 – 6.8.
Crop
Deep rooting crops have more areas to pull from.
Temperature
Temperature influences plant growth and P conversion.
Sources: mofga.org, agweb.com, Novozymes, MSU Extension, cropnutrition.com, and extension.umn.edu.