Do you understand the different forms of micronutrients?
Increased interest in micronutrients
When it comes to applying micronutrients, understanding the different forms that they can come in is very important to know. There is an increased interest in micronutrients with today’s growers. Many growers and advisors realize that these can be a major limiting factor in crop growth. In order to increase yields, the levels in the soil of micronutrients need to be monitored and properly placed. As well, more accurate soil testing through zone management has created the need to address micronutrients even more. There are agronomic advantages and disadvantages of each of the following forms of micronutrients. By understanding the different types of micronutrients, you can cater the right micronutrient product to the right field and operation.
To begin, micronutrient fertilizers (the metal cations including zinc, copper, manganese and iron) can be classified into two different categories. The first is called organic and the second is called inorganic.
Chelates
Chelates are considered to be organic molecules. The word “Chelate” is derived from a Greek word which when translated comes to mean “Claw.” What happens with chelates is that an organic molecule will cling on to the metal cation to form a ring like structure. Chelation is important because it makes the metal ions more available for uptake. Since Zn, Mn, Cu, and Fe are all cations they can react with Hydroxide (OH–) Ions which will make them unavailable and make your micronutrient fertilizer non effective. The “Claw” that is part of the chelates binds to the metal atom and protects it so that it can not link with OH– ions. This will allow the plant to be able to absorb the metal ion. One thing to note is that other positively charged ions such as Calcium or Magnesium can compete with the metal ion for binding. This metal ion can then be replaced, making the chelate ineffective in delivering the metal ion to the plant.
Advantages to Chelates are:
- Very soluble in water
- Readily available to plants
- It is available in many different ranges of soil pH
- With inorganic components in the soil, Chelates are less reactive with them
Disadvantages to Chelates are:
- Most expensive
- Since they are so readily available and very water soluble, it is easy to over apply and create toxicity problems
Oxides
Oxides are considered to be inorganic. For oxides, micronutrients such as Cu, Zn, Fe, and Mn will be bonded with oxygen to form oxides. Since the bond is so strong with oxygen it will not be soluble in water. The form will have to be converted in the soil. Once it is converted then it will be plant available. They are typically sold as powders or they are in a granular form. They are typically not effective in this granular form. Oxides usually have a higher analysis of the micronutrient compared to chelates. Where the oxide form could be effective is when it is applied as a powder form broadcasted and then incorporated.
Advantages to Oxides:
- Least expensive form of micronutrients
Disadvantages to Oxides:
- They are insoluble in a single crop season and therefore unavailable to a crop.
- Can be ineffective as a fertilizer source
- Must be finely ground to be effective in soils
- Often have to be applied months before a crop is to be put in to be effective
Oxysulfates
Are considered to be another inorganic source. These are a combination of oxides and sulfates. It stems from a micronutrient that has reacted with sulphuric acid. By having the oxide form be acidified by the sulfate form it will increase the water solubility of the micronutrient and make it more effective. They are usually sold in granular form.
Advantages of Oxysulfates:
- More cost effective then chelates, and still do a good job
- If small amounts are required you are better to go this route, since low amounts of chelated micronutrient doesnot seem to work as good since it is hard to achieve a uniform application.
Disadvantages of Oxysulfates:
- Low water solubility especially at higher ph levels.
- Greater risk for creating toxicity or damage if over applied
Sulfates
Sulfates are also considered to be inorganic. Sulfates such as Zinc Sulfate (ZnSO4) and manganese sulfate (MnSO4) are 100% water soluble. They are the most commonly used form for field crops. The nice thing about sulfates is that they can be applied to the soil or directly onto the leaf and stem of the plants. They are sold in crystalline or granular form.
Advantages of Sulfates:
- Can provide long term residual value.
- Can be foliar applied
Disadvantages of Sulfates:
- Greater risk for creating toxicity or damage if over applied
Remember there are some soil factors that can affect micronutrient availability and uptake. Some of these include:
- Soils low in organic matter (less than 2.0%) may have lower micronutrient availability.
- Soils with higher amounts of clay (fine texture) are less likely to be low in plant available micronutrients. Sandy soils(course texture) are more likely to be low in micronutrients.
- Soils that have very high levels of organic matter (greater than 30% organic matter to a depth of 30 cm) often have lowmicronutrient availability.
- Soil temperature and moisture are important factors. Cool, wet soils reduce the rate and amount of micronutrients thatmay be taken up by crops.
- As soil pH increases the availability of micronutrients decreases, with the exception of molybdenum.
Relative response of selected crops to micronutrient fertilisers.1
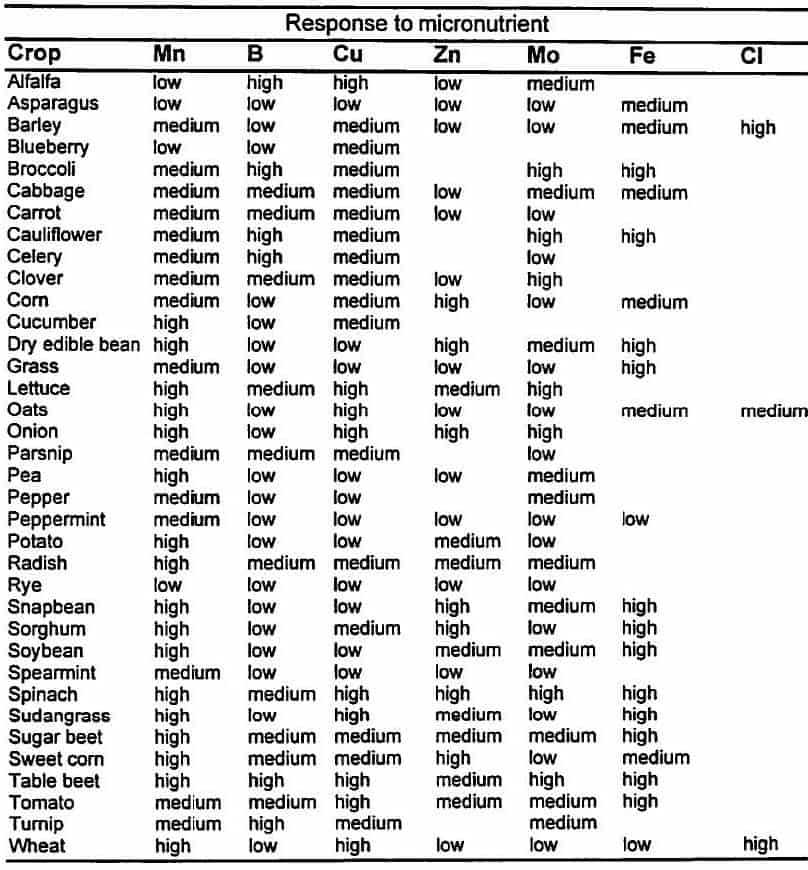
1Highly responsive crops will often respond to micronutrient fertiliser additions if the micronutrient concentrations in the soil is low. Medium responsive crops are less likely to respond, and low responsive crops do not usually respond to fertiliser additions even at the lowest soil micronutrient levels.
Sources: Iowa State University, agric.gov.ab.ca, smart-fertilizer.com